iAluRil®
IALURIL IS A MEDICAL DEVICE MANUFACTURED BY ASPIRE PHARMA,USED FOR THE TREATMENT OF CASES WHERE THE LOSS OF COMPONENTS OF THE BLADDER LINING CAUSES CHRONIC INFLAMMATION AND INFECTION.
iAluRil is a medical device presented in a 50ml pre-filled syringe with a Luer-Lock adapter or iAluadapter® for easy use.
iAluRil contains three active components: 800 mg/50 ml sodium hyaluronate (1.6%), and 1 g /50 ml sodium chondroitin sulfate (2%) and calcium chloride (0.87%).
iAluRil can be used for the treatment of:
Interstitial cystitis
Painful bladder syndrome
Recurrent urinary tract infections
Chemical-/radiation-induced cystitis (including BCG)
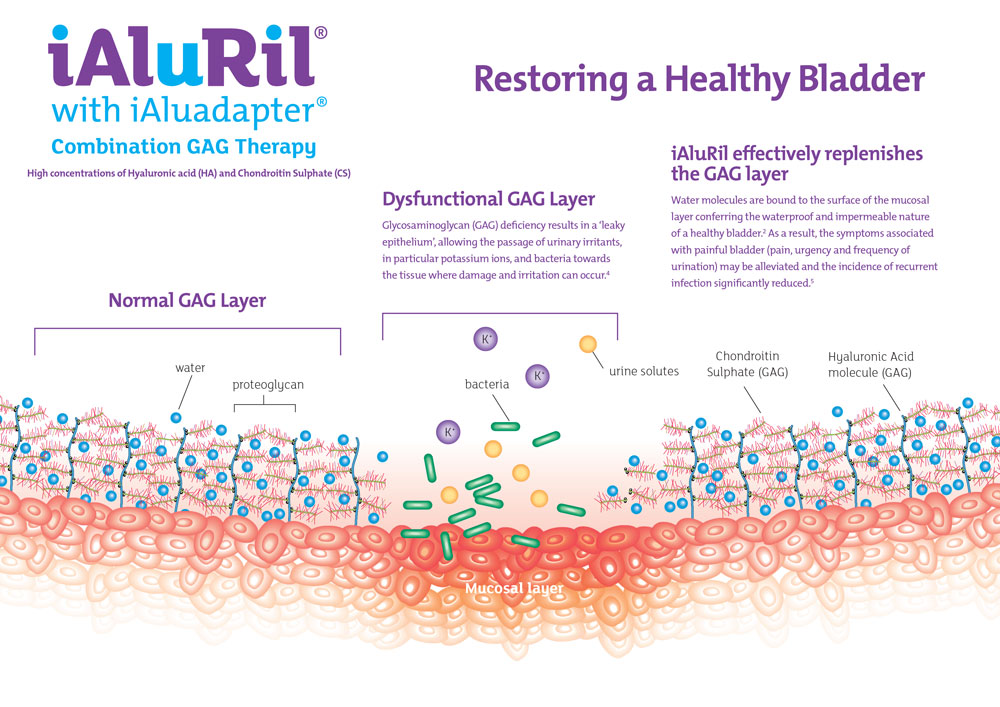
iAluRil® is the first intravesical GAG replacement therapy to combine one of the most abundant sulfated GAG molecules located on the bladder wall,2 chondroitin sulfate, with the most integral component of the GAG layer,3 hyaluronic acid. This combination is designed to facilitate faster and more effective restoration of the bladder epithelium.
How does iAluRil work?
In a healthy bladder there is a natural barrier that protects the bladder lining from the urine. This barrier is called the glycosaminoglycan (GAG) layer. If this barrier is damaged, urine comes into direct contact with the tissues of the bladder lining and over time can cause damage to these tissues.
This damage can lead to a range of problems that cause symptoms, such as pain, urgency (the need to go to the toilet immediately) and frequency (the need to go to the toilet more often). iAluRil contains two of the natural GAGs that form this barrier; hyaluronic acid (HA) and chondroitin sulfate (CS). By administering iAluRil directly into the bladder, these GAGs help to repair the damage to the GAG layer and restore the bladder’s protective coating, relieving these symptoms.
How is iAluRil administered?
iAluRil is administered directly into the bladder using a prefilled syringe that is passed through the urethra (the passage through which urine comes out when you go to the toilet) into the bladder. Before administering iAluRil, you will need to urinate and then the bladder can be emptied of all traces of urine by inserting a catheter and waiting for all of the urine to leave the bladder. When the liquid has been passed into your bladder it should be held there for at least half an hour. Once it can be held no more, the bladder can be emptied by urinating as normal.
This is a relatively painless and routine procedure, which may initially be carried out by a doctor or a nurse, however, many patients can be taught to do this themselves as it is very simple. If this is something that you feel you would prefer to do, then do discuss this with your doctor or nurse and they will be able to offer more advice.
How long does treatment last?
iAluRil treatment begins with a course of instillations given once a week for the first month. The instillations will then be given once every two weeks for the second month followed by once every month until the symptoms settle down.
Once the symptoms have improved, the interval between instillations can be increased slowly until you are happy that the regular doses are enough to keep symptoms under control. If the symptoms reoccur, the initial course of instillations can be repeated in order to settle the symptoms down again.
Are there any side effects?
iAluRil is contraindicated in patients with known hypersensitivity to the active substances or any of the excipients.
If you do experience any side effects discuss them with your nurse or doctor and between you a decision can be made whether to continue with or stop treatment.
FURTHER INFORMATION
Manufactured by IBSA FARMACEUTICI ITALIA SRL
Under license from NTC S.r.l
1) iAluRil Prefill Patient Information. 2) Hurst RE et al. Functional and Structural Characteristics of the Glycosaminoglycans of the Bladder Luminal Surface. J Urol 1987; 138 (2): 433-437. 3) Stryer L. Biochemistry – 4th Edition. W.H. Freeman & Company; 1995. 4. Parsons CL et al. Abnormal Sensitivity to Intravesical Potassium in Interstitial Cystitis and Radiation Cystitis. Neurourol Urodyn 1994; 13(5): 515-520. 5. Damiano R et al. Prevention of Recurrent Urinary Tract Infections by Intravesical Administration of Hyaluronic Acid and Chondroitin Sulfate: a Placebo-Controlled Randomised Trial. Eur Urol 2011; 59(4): 645-651. 2011; 59(4): 645-651.
Prescription-only.
For any queries on availability of this product outside of the Republic of Ireland, please contact Aspire Pharma UK for more information info@aspirepharma.co.uk